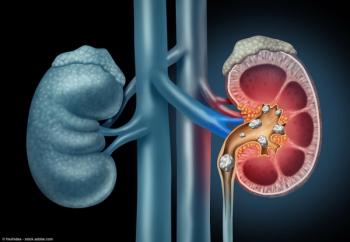
"Suctioning technology for mini-PCNL is an improvement on relying on passive outflow," says Mantu Gupta, MD.

"Suctioning technology for mini-PCNL is an improvement on relying on passive outflow," says Mantu Gupta, MD.

The approval is supported by data from the phase 3 MK-3475A-D77 trial.

The Altaviva device is a minimally invasive implantable tibial neuromodulation therapy for patients with urge urinary incontinence.

According to the authors, these findings suggest that FloStent could serve as an effective interim solution for men who are awaiting TURP.

Recent data suggests a lack of knowledge among primary care providers on the value of prostate specific antigen testing, particularly for patients in high-risk groups.

According to the authors, these findings suggest that biparametric MRI could become the new standard of care for prostate cancer diagnosis.

Two-year outcomes from the ASPIRE trial continued to show that the SURE procedure led to significantly reduced health care consumption events compared with ureteroscopy.
The NDA is supported by data from a phase 3 trial, which demonstrated a 75% complete response rate at 3 months.

Siamak Daneshmand, MD, shares his thoughts on the impact of the gemcitabine releasing system for clinical practice.

The registrational trial is aiming to expand the indications for Illuccix and Gozellix to include use in prostate cancer diagnosis.

"As urologists, we should be proactive and creative as we find new ways to attract, retain and train the next generation," writes Michael S. Cookson, MD, MMHC, FACS.
CG Oncology plans to initiate a BLA submission with the FDA in Q4 of 2025.

The AUA has released updated guidelines for recurrent UTIs in women, emphasizing patient-centered care, nonantibiotic options, and improved clinical judgment.

Hong Truong, MD, MS, details key findings on referrals for genetic counseling among patients with kidney cancer who meet the criteria for genetic evaluation.
The LEGEND trial is evaluating detalimogene voraplasmid in patients with high-risk non–muscle invasive bladder cancer.

The report showed rising incidence rates and a slowing of mortality declines in addition to persistent racial disparities.

The new guidelines provide evidence-based recommendations for treating patients with traumatic injuries to the kidneys, bladder, ureters, urethra, and genitalia.

The approval is supported by findings from an open-label, single-arm, phase 3 trial.

The findings highlight a lack of awareness on the signs and symptoms of prostate cancer, as well as common misconceptions about testicular cancer.

"We have received numerous complaints about the PA and predetermination process. We all wish we could answer this differently and definitively," write Jonathan Rubenstein, MD, and Mark Painter.