
Overall, 71% of patients had no fragment greater than 2 mm following treatment.

“We are witnessing the dawn of teletreatment,” says Jihad Kaouk, MD, FACS.
Two recent Clinical Forum discussions sought to examine how emerging therapeutic options and evolving treatment standards in non–muscle invasive bladder cancer affect clinical practices and patient outcomes.

Three recent expert-led Clinical Forums explored current challenges and advancements in the management of non–muscle invasive bladder cancer.

Data showed a significant association between the C allele of the SNP-765G>C of the PTGS2 gene and BPH.

Data from the pivotal CONTINENCE trial showed encouraging efficacy of SCONE neuromodulation therapy.

The first patient in the trial will be receiving 67Cu-SAR-bisPSMA in combination with enzalutamide.
The sBLA is supported by data from cohort B of the pivotal QUILT-3.032 trial.

Christian Gratzke, MD, explains why an app shows promise for patients with lower urinary tract symptoms.

"The goal of the PRECISION study is to ensure that treatment with 3 radiation doses does not lead to greater side effects than those observed in the PACE-B study," says Professor Duncan B. McLaren, MBBS.

Jonathan Rubenstein, MD, and Mark Painter answer a question regarding coding for use of the second-generation Calyxo CVAC system.
“The technology in the field of stone management is exciting, and I think it offers patients a lot more options than what they've had in the past,” says Brian Stork, MD.

An approach to WVTT involving 1 treatment per prostate lobe was associated with decreased post-operative complications.

This approval marks the first clearance of a stapler designed for single-port robotic surgery in the US.

"Building on decades of research into the genetic markers of prostate cancer, our study shows that the theory does work in practice—we can identify men at risk of aggressive cancers who need further tests and spare the men who are at lower risk from unnecessary treatments," says professor Ros Eeles.
In February 2025, the FDA recommended a removal of the black box warning related to an increased risk of adverse cardiovascular outcomes for all testosterone products. This recommendation has important implications for urologists and the patients they treat.

At week 12, patients in the URO-902 cohorts demonstrated significantly greater improvements in daily microuritions vs placebo.

"For every 1 unit increase in whole body tumor SUVmean, there was a 12% decrease in the risk of an rPFS event and a 10% decrease in the risk of death," says Phillip H. Kuo, MD, PhD, FACR.
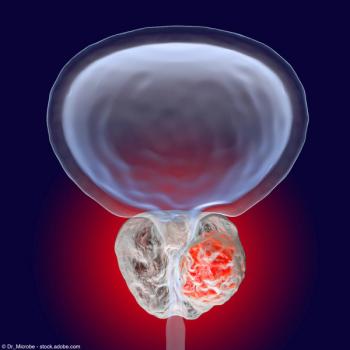
"This finding reinforces PROSTOX ultra as a true measure of the biological response to radiation, independent of treatment era or technique that can identify the safest course of treatment to avoid toxicity," says Amar U. Kishan, MD.

A lookahead of the notable FDA decisions and conferences slated for Q2 2025.

"The biggest message from this study is that 60% can expect resolution of UUI, simply by fixing the prolapse and placing a sling, which is not normally indicated for UUI," according to Christina M. Mezes, DO, and Catherine A. Matthews, MD.

“The differences in patient experience between UroLift System and Rezūm, particularly in terms of early recovery, sexual function, and overall satisfaction, are key considerations for both clinicians and patients selecting a treatment path," says Mark Rochester, MD, FRCS.

"The Inflation Reduction Act was a major step in the right direction. The question now is going to be, are commercial insurers going to follow Medicare and enact the same cap that they have as well," says Benjamin Pockros, MD, MBA.

"The combination demonstrated clinical activity and an acceptable safety profile in the perioperative setting for locally advanced, non-metastatic clear cell RCC," says Mehmet Asim Bilen, MD.

The company plans to initiate the phase 2 monotherapy trial by mid-year.

"In terms of making it a safe, highly reliable operation, if you're comfortable with slings and sphincters, you are very well positioned to learn this surger," says Kiran Sury, MD.

"Glean will enable patients to void naturally, without catheters in place. I believe this advance will yield a better test that mimics actual symptoms," says Bilal I. Chughtai, MD.

"We must lobby to maintain our current funding levels and push for increased support to continue driving progress in cancer care," says Michael S. Cookson, MD, MMHC, FACS.

“The data presented at EAU25 add to the growing body of evidence supporting the CVAC System as the emerging standard of care in kidney stone removal,” says Brett Johnson, MD.

A recap of the FDA submissions and regulatory decisions in urology from March 2025.