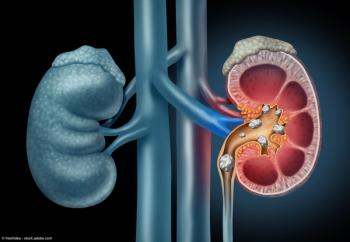
"I think the patients that benefit the most are patients that have certain types of stones that potentially are much harder to treat with some of the more minimally invasive types of procedures," says John Michael DiBianco, MD.

"I think the patients that benefit the most are patients that have certain types of stones that potentially are much harder to treat with some of the more minimally invasive types of procedures," says John Michael DiBianco, MD.

Data from a real-world evidence study of the Leva System were recently published in JMIR Formative Research, supporting the safety and effectiveness of the program in the treatment of patients with urinary incontinence.
Patient recruitment for the phase 2 trial is expected to begin by the end of 2024.

With this designation, the development process for 9MW2821 can benefit from prioritized engagement with China’s Center for Drug Evaluation as well as expedited review and approval.

The phase 1/2 trial is assessing the safety, tolerability, radiation dosimetry, and preliminary anti-tumor activity of 225Ac-rhPSMA-10.1 in men with mCRPC.
"We propose a new classification system, which we believe will provide a more consistent and standardized approach to classification and treatment of PD," says Landon Trost, MD.

"By helping them see what could be going on with their health and knowing when to reach out for help, we think that's a fantastic way to begin to change the paradigm when it comes to men and prostate cancer in the US," says Motolani Adedipe, PhD, DPh, MSc.

The median PFS with PSMA PET/CT-guided MDT was 33.2 months, vs 13.8 months with choline PET/CT-guided MDT.
Data from the study showed that 45% of biopsies with benign results or low-grade cancers could be reduced overall by using Stockholm3 compared with PSA.

"If we don't take the time to speak to trainees, an already vulnerable population, we're missing an opportunity to help them early," says Andrew M. Harris, MD.

“Our data suggest that an MRI can show suspicious lesions based on size and markers of tumor aggression, which may help doctors differentiate a treatment path for these patients," says Kiran R. Nandalur, MD.

"The scale of that linkage and the manner with which we were able to do it is really exciting and opens the door for a lot of future discovery," says Michael S. Leapman, MD, MHS.

The prognostic test has now been validated in patients who have undergone active surveillance, radiation therapy, or had a radical prostatectomy.

In preclinical studies, META-001-PH demonstrated the ability to reduce urinary oxalate excretion by up to 80%.

Telix must resubmit the BLA with remediations in order to advance the application to full review, which the company expects to be able to complete within approximately 90 days.

“So, rather than waiting for men to proactively seek out mental health supports once they’ve been diagnosed with prostate cancer, we should be offering supports at the time of diagnosis and throughout treatment," says Kerri Beckmann, PhD.

"We wanted to look at the importance of genetic testing in patients with GU cancer and the impact that it has on their treatment decision-making, and the pathways that we put them on," says Mouneeb Choudry, MD.

Aspargo plans to file a new drug application with the FDA to support regulatory approval of ASP-001 for erectile dysfunction in the United States.

"One of the main conclusions that we can take from our study is that although the current classification systems are somewhat accurate, they are far from being perfect," says Félix Guerrero-Ramos, MD, PhD, FEBU.

"We can individualize the access and the way we do the operation based on the patient's characteristics, and that hasn't happened in a very long time," says Ketan K. Badani, MD.

The grants will support research evaluating ATAD2 in advanced prostate cancer and 6MP in hereditary leiomyomatosis and renal cell cancer.

"Teixobactin, and more broadly, the iChip approach to novel antibiotic discovery, is an exciting development in infection control," write Philip Abbosh, MD, PhD, and Kate Gessner, MD, PhD.

The CPT code 76377 may be used for ProstatID when the software is used in conjunction with a suitable CT, MRI, or a transrectal ultrasound.

"This study demonstrates that for up to 10 years after diagnosis, approximately 50% of men with low-risk prostate cancer can safely remain on AS, without cancer progression or treatment, since less than 1% died of prostate cancer," writes Badar M. Mian, MD.

A decision on the NDS for testosterone undecanoate is expected in Q2 of 2025.

“I think the use and the appropriateness of these agents along the patient journey is also coming into question,” says Brian T. Helfand, MD, PhD.

Data showed a patient-level detection rate of 93% with flotufolastat F 18 in African American patients.

"The CF for 2025 is proposed to decrease to $32.36 from $33.29 in 2024. This is a decrease of 2.80%," write Jonathan Rubenstein, MD, and Mark Painter.

"This study was looking at the incidence of cognitive impairment and/or manual dexterity disorder diagnoses in men who underwent an artificial urinary sphincter after treatment for prostate cancer," says Jacqueline Zillioux, MD.

Data showed that Hispanic patients were consistently underrepresented in clinical trials, while Black participants tended to be overrepresented.