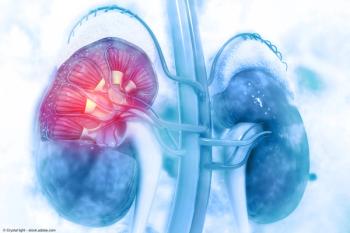
Patients with advanced renal cell carcinoma treated with TKIs in the second or third line following lenvatinib treatment showed modest activity, highlighting the need for improved treatment options.

Kristie L. Kahl is vice president of content at MJH Life Sciences, overseeing CURE®, CancerNetwork®, the journal ONCOLOGY, Targeted Oncology, and Urology Times®. She has been with the company since November 2017.
She is a graduate of Rider University, where she acquired a Bachelors of Art in journalism, as well as a graduate of Temple University, where she received her Masters of Science in Sports Management.
Follow Kristie on Twitter at @KristieLKahl, or email her at kkahl@mjhlifesciences.com.

Patients with advanced renal cell carcinoma treated with TKIs in the second or third line following lenvatinib treatment showed modest activity, highlighting the need for improved treatment options.

Ten-year follow-up data from the GETUG-AFU 18 trial showed that in patients with high-risk prostate cancer, survival outcomes were improved when combining a higher dose of radiation therapy with long-term use of androgen deprivation therapy.
The patents apply exclusively to the Ga-68 HBED-PSMA-11 production kit (Locametz), developed by Advance Accelerator Applications.

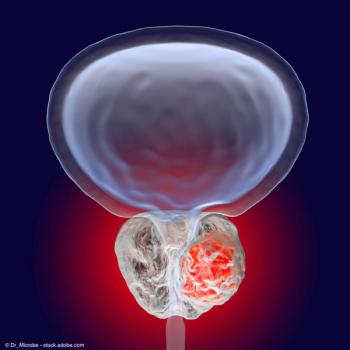
The application is based on results from the phase 3 CheckMate-901 trial.

Although low, men experienced a higher risk for erectile dysfunction and hypogonadism after being prescribed semaglutide for weight loss; however, further questions remain.
Further, men with Peyronie disease treated with PRP injections demonstrated a 25% reduction in curvature at a median of 10 degrees after 6 months.

At 5 years from CCH injection, curvature and plaque volume were associated with patient satisfaction in the treatment of their Peyronie’s disease.

Findings showing an increased risk for UTIs highlight the importance of follow-up and counseling of women during the initiation of anti-androgen therapy.

In a retrospective study of almost 500 men, the use of hyaluronic acid filler for penile girth enhancement appeared safe with limited adverse events.

Albeit a rare cancer, the understanding of penectomy options in penile cancer is important for physicians, as more data are also needed, according to Senthooran Kalidoss.

Compared with retail and Medicare pricing, the use of discount listings can induce significant cost savings, improving patient adherence to vaginal estrogen treatment.
At 4 years post-surgery, men receiving Rezum had preserved and/or improved sexual function and better lower urinary tract symptoms secondary to benign prostatic hyperplasia.
The application is based on results from the phase 3 CheckMate-901 trial.

Frontline therapy with nivolumab plus gemcitabine-cisplatin, followed by nivolumab maintenance, led to improved overall and progress-free survival in patients with unresectable or metastatic urothelial carcinoma.
“Future studies should focus on patient selection and the identification of positive predictive markers of response, more so than treating all comers, which several studies have shown has not really worked here or they acknowledge it,” says Julie N. Graff, MD.
“This is a pivotal phase 3 trial that I think is going to be practice changing to the urology community,” said Brian Shuch, MD.
Adding apalutamide to ADT with or without abiraterone acetate/prednisone significantly prolonged PSA PFS in men with biochemically relapsed prostate cancer, according to extended follow-up from the phase 3 PRESTO study.
“These results further support nivolumab as a standard of care for high-risk MIUC and MIBC after radical resection,” said Matthew Milowsky, MD.

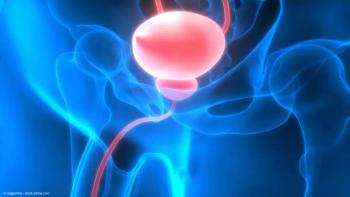
Single-dose URO-902 appeared to be safe and effective in treating women with overactive bladder and urge urinary incontinence, according to the final analysis of a phase 2a trial.

The results may help guide the decision between a partial and radical nephrectomy in patients with stage T1b to T2 renal cell carcinoma.

The deep PSA responses with the darolutamide regimen were found to be correlated with improved overall survival and a prolonged time to PSA progression.
Irrespective of response to first-line platinum-based chemotherapy, frontline maintenance therapy with avelumab plus best supportive care (BSC), compared with BSC alone, prolonged overall and progression-free survival in patients with advanced urothelial carcinoma.
"Dose reduction for patients with small body size may alleviate skin AEs without sacrificing oncological outcomes,” said Kyo Togashi.
“So I think what we can take away from this study is that just because your patient [has a higher BMI], doesn't mean that they're a poor candidate for a partial nephrectomy,” said Lachlan Shiver.

Pembrolizumab monotherapy continued to demonstrate durable complete responses, while rates of upstaging at the time of radical cystectomy were consistent with previous findings in the KEYNOTE-057 trial in patients with BCG-unresponsive NMIBC.
As stereotactic ablative radiation therapy enters further into the treatment space for metastatic RCC, integration of the approach is vital for physicians to focus on, according to Raquibul Hannan, MD, PhD.

Compared with data from the KEYNOTE-057 study, the use of pembrolizumab monotherapy in the real-world setting was similar in the treatment of patients with high-risk non-muscle invasive bladder cancer.
The combination use of PSMA-targeted radionuclide therapy tailored by the individual patient and dose may show promise in the future treatment of men with metastatic castration-resistant prostate cancer.
“There is a critical unmet need for efficacious bladder-sparing therapies for patients with BCG-unresponsive bladder cancer,” said Roger Li, MD.

The combination use of cabozantinib plus nivolumab and ipilimumab reduced the risk for disease progression by 27% in patients with previously untreated advanced renal cell carcinoma.
Published: February 12th 2021 | Updated:

Published: September 21st 2020 | Updated:
Published: November 18th 2023 | Updated:

Published: September 21st 2020 | Updated:

Published: December 1st 2020 | Updated:

Published: December 3rd 2020 | Updated: