
“A key point is the supply chain is very fragile, and there's not really a good financial incentive to make these drugs," says Ruchika Talwar, MD.

“A key point is the supply chain is very fragile, and there's not really a good financial incentive to make these drugs," says Ruchika Talwar, MD.

The study will evaluate the investigational drug product ONC175 (orellanine) in patients with metastatic clear cell or papillary renal cell carcinoma.

The trial is assessing the safety and tolerability of adaptive radiation therapy with concurrent sacituzumab govitecan in patients with MIBC.

“The study suggests that exercise would be an effective intervention for men with prostate cancer who express concern about sexual dysfunction, and that exercise medicine should be considered a key part of their treatment,” says Daniel Galvão, PhD.

"This certainly does feel like the beginning of the light at the end of the tunnel coming out of the BCG shortage," says Chad A. Reichard, MD.
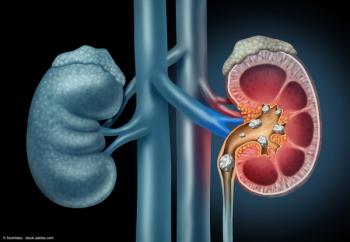
The overall prevalence of kidney stones remained stable, with a notable increase observed among women.

The 52-week study plans to enroll 16 patients across 4 sites in the US.

The mean absorbed radiation dose to tumors was 8.9 Gy/GBq, compared with 0.27 Gy/GBq in the kidneys and 0.13 Gy/GBq in the salivary glands.

"The relaxation of the restrictions that started during COVID are critically important for continued access," says Mark T. Edney, MD.

There are also 60 sites in the process of launching the EAP.

The method involves local delivery of the antibiotic gentamicin directly into the bladder tissue.
This bill would cut funds for research projects in disease states such as prostate cancer, bladder cancer, kidney cancer, and interstitial cystitis.

“We might need to do a better job in general about education of the importance of follow-up,” says Christina B. Ching, MD.

"A 14-month delay in disease progression is a very meaningful end point to our patients, because that basically means delay in symptoms, delay in fractures, [and] delay in pain in the castration-resistance setting," says Neeraj Agarwal, MD, FASCO.

"I'm so excited about them finally removing this boxed warning," says Helen L. Bernie, DO, MPH.

"The findings from this study will help us understand sunobinop’s potential as a possible new treatment option for this chronic disorder,” said Craig Landau, MD.

The goal of the trial is to improve quality of life by identifying patients who can safely reduce or avoid treatment with hormone therapy.
“For someone that might be looking at getting into sacral neuromodulation, and has had some reluctance in the past, this does simplify the procedure somewhat," says Colin Goudelocke, MD.

"Considering that almost all patients in the combined treatment group achieved testosterone recovery at 1 year, the results support that this short term (intermittent) combined approach is an optimal option in selected patients with metachronous oligorecurrent hormone-sensitive prostate cancer," the authors wrote.

“Now, at 1 year, the 1-hour pad test has been done again, as it's part of the protocol, and she suffered 4 g of incontinence on this test, opposed to 125 g at the beginning,” says Emmanuel Chartier-Kastler, MD, PhD.

“Interestingly, the patients that were more likely to follow-up were those patients that were seen and established within our urology clinic,” says Christina B. Ching, MD.

“Five years after telehealth suddenly became a routine part of care for most Americans, we show that it has not led to runaway utilization or spending. This kind of in-depth analysis can inform its future," says Chad Ellimoottil, MD, MS.

"At 3 months, those patients had a 79% complete response rate, which was durable in 74% at 6 months and 60% at 9 months," says Jacob A. Moyer, BS.

The detection rate ranged from 44% to 58% on day 0 and 58% to 80% on day 1.

“What we hope overall is that this is a biological study that might inform therapeutic development for this disease,” says David A. Braun, MD, PhD.

"The preliminary results of that study, which is a phase 1/2 dose escalation and dose expansion trial, were presented by Professor Ben Tran last year at the ENA meeting1 and showed a very favorable toxicity profile compared to erdafitinib," says Gopa Iyer, MD.

The safety review committee recommended that the phase 2 portion proceed with the 8 GBq dose.

The average life expectancy was 80.3 years among men with a total motile sperm count over 120 million, compared with 77.6 years among men with a total motile sperm count ranging from >0 to 5 million.

The ARID II trial is assessing the safety and effectiveness of the device in men who are scheduled to undergo robotic-assisted radical prostatectomy.

The median OS was 34 months with 177Lu-PSMA-617 plus enzalutamide compared with 26 months with enzalutamide alone.