
PT217 is currently under investigation in the phase 1/2 SKYBRIDGE trial in patients with neuroendocrine cancers.

PT217 is currently under investigation in the phase 1/2 SKYBRIDGE trial in patients with neuroendocrine cancers.
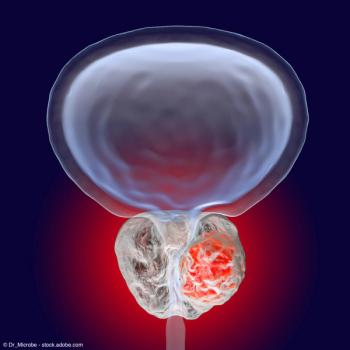
Data showed that all patients achieved a PSA decline of at least 50%.
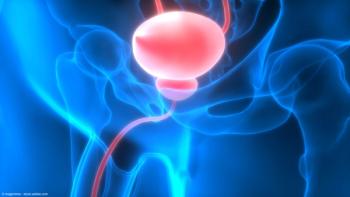
Ferring has announced the opening of the first clinical trial sites for 2 studies within the ABLE clinical trial program as well as the launch of the phase 1/2 LUNAR trial.

“The combination of pembrolizumab and chemotherapy presents a promising new treatment approach for these challenging-to-treat, rare cancers and could be a major breakthrough for patient care,” says Arnold I. Chin, MD, PhD.
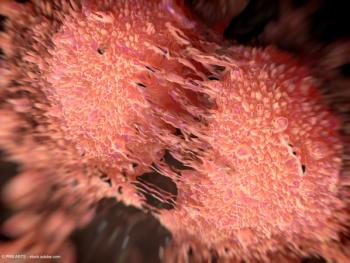
The phase 2 CELLVX-230 trial is exploring the potential of FK-PC101 to delay or prevent prostate cancer recurrence following prostatectomy.
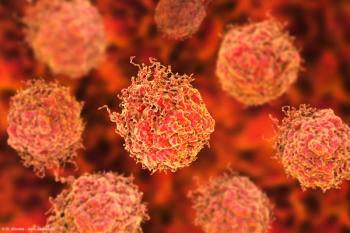
“Our study highlights the potential of epigenomic profiling from a simple blood draw to provide a real-time, non-invasive readout of PSMA expression that corresponds with treatment response," says Jacob E. Berchuck, MD.

“The median DOR of 47.8 months in patients who achieved complete response with JELMYTO provides evidence of robust durability in maintaining control of low-grade UTUC over an extended period,” says Phillip Pierorazio, MD.

The study showed significant reductions in both anxiety and depressive symptoms following the intervention.

Data from the CAPItello-281 trial also showed a trend toward an improvement in overall survival.
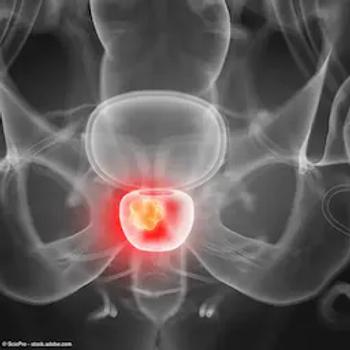
The trial is evaluating the diagnostic performance of copper Cu 64 PSMA I&T PET/CT in men with suspected biochemical recurrence of prostate cancer following treatment with curative intent.

In the recent news release, Koelis reported that the final patient was enrolled in the VIOLETTE study in September 2024.

The sNDA is supported by data from the pivotal phase 3 ARANOTE trial.

“Prostate cancer patients with life expectancies of less than 5 or 10 years were being subjected to treatments that can take up to a decade to significantly improve their chances of surviving cancer, despite guidelines recommending against treatment," says Timothy J. Daskivich, MD.
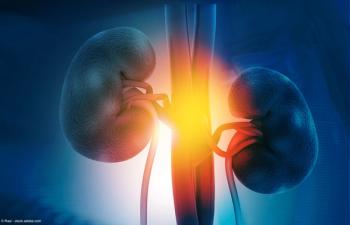
Initial results on ADI-270 are anticipated for the first half of 2025.

At the time of data cutoff, 71% of patients achieved a complete response following treatment.

The primary objective is to compare the detection rate of sites of prostate cancer recurrence between the 2 agents.

Data demonstrated wide variation in the proportion of patients recommended for testing and showed that barriers to testing remain.

Final completion of the ECLIPSE trial is anticipated for February 2029.

"The Stone Clear device provides patients with a non-invasive option to reduce their residual fragment stone burden in the clinic environment while being fully awake," says James E. Lingeman, MD.

The phase 1/2a SECuRE trial is evaluating the safety and efficacy of 67Cu-SAR-bisPSMA in the treatment of patients with mCRPC.
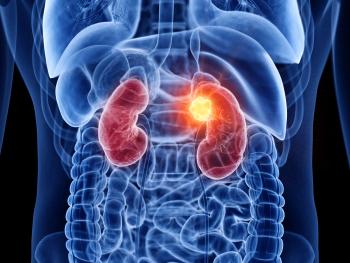
The sensitivity of 99mTc-sestaMIBI SPECT/CT was 97%, and the specificity was 53.8%.

In total, 33.3% of patients with a VUS also had positive lymph node involvement and required adjuvant ADT compared with only 16.2% of patients who did not have a VUS.

"Together, the data point to the exciting potential for this combination regimen to be used to treat patients with muscle invasive bladder cancer," says Roger Li, MD.

“It is critical that urologists understand when to order germline testing, are comfortable with actually ordering these tests, and integrate medical geneticists when appropriate," says Aditya Bagrodia, MD, FACS.

“Our study is distinguished by long follow-up, out to 12 years, looking at a broad spectrum of key complications," says Joseph M. Unger, PhD.
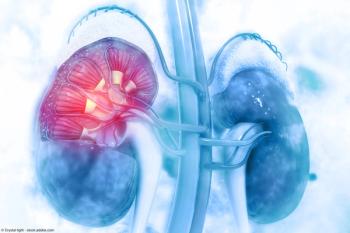
Overall, 76% of patients with a CD70 TPS of at least 50% achieved a reduction in tumor burden.
The decision was based on an interim readout from the trial, which showed that ONCT-534 did not lead to any clinically meaningful improvement of disease.
At the end of the induction phase, 86% of patients had achieved a complete response.

Patients with high P-CARE scores had an increased risk of any, metastatic, and fatal prostate cancer compared with patients with median P-CARE scores.

The observational LOBSTER study has already enrolled 303 patients and collected 479 samples.