The approval is supported by findings from the phase 3 EV-302 trial.

The approval is supported by findings from the phase 3 EV-302 trial.
“This study is a breakthrough because it is the first trial to show that a non-hormonal drug can induce durable complete remissions in recurrent prostate cancer patients with BRCA2 mutations—one of the most aggressive subtypes of this disease,” says Emmanuel S. Antonarakis, MD.

The fast track designation for 64Cu-SAR-bisPSMA is supported by findings from Clarity’s ongoing clinical program for the imaging agent.
Any stone fragmentation was achieved in 88% of patients.

A full market release of the HYDROS System is expected within the current quarter.
"Overall, we found that patients with recurrent RCC following adjuvant IO continued to have responses to different types of first-line systemic therapy," says Talal El Zarif, MD.

The approval for enfortumab vedotin is supported by findings from the global phase 3 EV-301 trial and the phase 2 EV-203 bridging trial in Chinese patients.
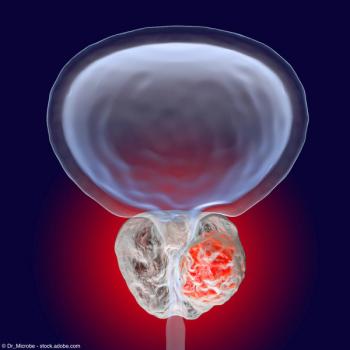
The phase 1 trial is assessing the safety and tolerability of MOMA-313 as both a monotherapy and in combination with olaparib in patients with HR-deficient advanced or metastatic solid tumors.
"The updated guideline includes numerous new, important recommendations that will enhance the ability of urologists to provide optimal, state-of-the-art care for infertile males,” says Robert E. Brannigan, MD.

"Combining PRIMARY and PI-RADS scores could be a game-changer in how prostate cancer is diagnosed," says Louise Emmett, MBChB, FRACP, MD.

Per Kaplan-Meier estimates, 95.6% of patients who underwent SURE were free of health care consumption events by 12 months, compared with 83.5% of patients who underwent URS.
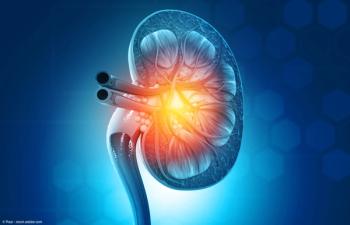
“The challenge now is to understand how these key aspects of mitochondrial metabolism become activated, why they stimulate metastasis, and whether we can safely block them,” says Ralph J. DeBerardinis, MD, PhD.

The NDA for UGN-102 is primarily supported by findings from the phase 3 ENVISION trial.

Data from a real-world evidence study of the Leva System were recently published in JMIR Formative Research, supporting the safety and effectiveness of the program in the treatment of patients with urinary incontinence.
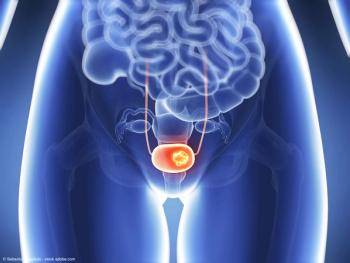
Patient recruitment for the phase 2 trial is expected to begin by the end of 2024.
With this designation, the development process for 9MW2821 can benefit from prioritized engagement with China’s Center for Drug Evaluation as well as expedited review and approval.

The phase 1/2 trial is assessing the safety, tolerability, radiation dosimetry, and preliminary anti-tumor activity of 225Ac-rhPSMA-10.1 in men with mCRPC.

"We propose a new classification system, which we believe will provide a more consistent and standardized approach to classification and treatment of PD," says Landon Trost, MD.
The median PFS with PSMA PET/CT-guided MDT was 33.2 months, vs 13.8 months with choline PET/CT-guided MDT.
Data from the study showed that 45% of biopsies with benign results or low-grade cancers could be reduced overall by using Stockholm3 compared with PSA.

“Our data suggest that an MRI can show suspicious lesions based on size and markers of tumor aggression, which may help doctors differentiate a treatment path for these patients," says Kiran R. Nandalur, MD.

The prognostic test has now been validated in patients who have undergone active surveillance, radiation therapy, or had a radical prostatectomy.

In preclinical studies, META-001-PH demonstrated the ability to reduce urinary oxalate excretion by up to 80%.

Telix must resubmit the BLA with remediations in order to advance the application to full review, which the company expects to be able to complete within approximately 90 days.

“So, rather than waiting for men to proactively seek out mental health supports once they’ve been diagnosed with prostate cancer, we should be offering supports at the time of diagnosis and throughout treatment," says Kerri Beckmann, PhD.

Aspargo plans to file a new drug application with the FDA to support regulatory approval of ASP-001 for erectile dysfunction in the United States.

The grants will support research evaluating ATAD2 in advanced prostate cancer and 6MP in hereditary leiomyomatosis and renal cell cancer.

The CPT code 76377 may be used for ProstatID when the software is used in conjunction with a suitable CT, MRI, or a transrectal ultrasound.

A decision on the NDS for testosterone undecanoate is expected in Q2 of 2025.

Data showed a patient-level detection rate of 93% with flotufolastat F 18 in African American patients.