
"The reported bpMRI-based AI model detected the majority of locally recurrent prostate cancer after radiotherapy," the authors wrote in their study conclusion.

"The reported bpMRI-based AI model detected the majority of locally recurrent prostate cancer after radiotherapy," the authors wrote in their study conclusion.
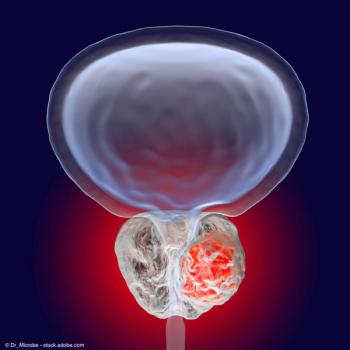
Patients who underwent treatment with SBRT demonstrated a 5-year disease control rate of 96%, compared with 95% among patients who underwent conventionally fractionated radiation therapy.

In the survey, 81% of residents in their final year of training reported that they sometimes, often, or always experienced feelings of burnout during their training.

The positioning system would allow patients with cancer to undergo radiation therapy in an upright, seated position.
About 1 of every 5 patients with stage I seminoma relapses; however, “the level of evidence supporting the use of currently defined risk factors in decision making is low,” wrote lead author Thomas Wagner, MD, and coinvestigators.

The inclusion of iTind is reflected in a newly added statement on temporary implanted prostatic devices.

If replicated with further study, the finding potentially expands noninvasive fertility treatment options and may minimize the need for expensive reproductive technology, according to lead author Scott D. Lundy, MD, PhD, of Cleveland Clinic’s Department of Urology.

“Implementation of the most recent PI-RADS update did not improve the incongruence in prostate cancer grade assessment between MRI/ultrasound fusion targeted biopsy and surgery,” the authors wrote.

"It is the right time and I have been fortunate to have such great people to work with locally and nationally," a urologist writes.
"It is erdafitinib’s time to shine," says Arlene O. Siefker-Radtke, MD.

The findings suggest that drug therapies targeting the NOTCH1 gene may serve as an alternative treatment option for patients with kidney cancer in whom surgery is not recommended.

The PSE test was shown to improve the predictive accuracy of a standard PSA test from 55% to 94%.

The reimbursement code for flotufolastat F 18 will be effective October 1, 2023.

“A hybrid telehealth approach expands access to specialized care, reduces travel burdens, promotes timely interventions, and enables continuous management of health conditions,” writes Kara Hartl, MD.

The addition of SBRT to upfront abiraterone acetate and prednisone (AAP) reduced the risk of disease progression or death by 65% compared with AAP alone in patients with oligometastatic castration-resistant prostate cancer.

“[These] results imply that prostate cancer survival could be improved, especially in historically under-served groups, by improving the timeliness of access to medical care," says Sean Clouston, PhD.

"At some point, it will make sense, and it will be obvious to me and to my colleagues that it is time," a urologist writes.
UCSF is West Coast leader for the innovative, minimally invasive treatment for prostate cancer patients.
The EV-302 trial is the confirmatory trial for the FDA’s accelerated approval of pembrolizumab plus enfortumab vedotin for the first-line treatment of patients with locally advanced or metastatic urothelial carcinoma.

"We all need to revisit meaningful methods for ongoing assurance of competency specific to our fields of medicine and surgery across the physician career span," writes Hal H. Atkinson, MD, MS.

Proper coding for percutaneous nephrolithotomy includes code 50081.

Liposomal tacrolimus led to improvements in hematuria, bleeding sites on cystoscopy, microscopic urine analysis for red blood cells, and urinary symptoms.

"To form a connection between the witness and the jury and establish the witness’ credibility, the witness needs to be the star," writes Austin M. Richards, Esq.
“The ADVANCED-2 trial in NMIBC is an exciting opportunity to build on the favorable anti-tumor and safety data of TARA-002 presented earlier this year," says Gautam Jayram, MD.

There have been several recent regulatory updates related to cybersecurity in health care.

The inclusion of PAE in the American Urological Association BPH Guidelines offers a non-surgical alternative that can provide long-term relief with minimal downtime and fewer complications, according to the United Urology Group.

"As the number of women urologists continues to increase, the field of urology must pay attention to their needs and professional satisfaction as they will constitute an increasing proportion of the future urologic work force," writes Lourdes Guerrios Rivera, MD, MSc.

Patients with mCRPC will be randomly assigned 2:1 to receive masofaniten/enzalutamide or enzalutamide alone.

"Training with aging faculty teaches important lessons. And it is the right balance of the lessons with a young ambitious trainee that has molded me into the product I have become," the urologist writes.

The supplemental new drug application is supported by findings from the LITESPARK-005 trial.