
"The EOM may facilitate success for its oncology participants by reducing reporting burdens, simplifying requirements, and offering potentially large bonus payments for lowering costs," writes Robert A. Dowling, MD.

"The EOM may facilitate success for its oncology participants by reducing reporting burdens, simplifying requirements, and offering potentially large bonus payments for lowering costs," writes Robert A. Dowling, MD.
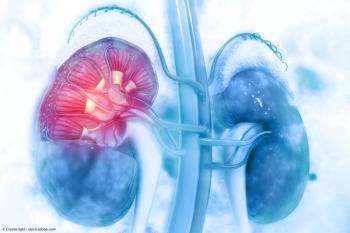
A Cleveland Clinic study found that TKIs in the neoadjuvant setting are associated with greater use of partial nephrectomy when treating patients diagnosed with renal masses in a solitary kidney.
The published findings show the viability of using a transperineal ultrasound system as a means to monitor intra-fractional prostate motion for prostate stereotactic body radiotherapy.

“Our findings show that AI chatbots provided accurate information with little misinformation. However, the information was provided at a college reading level and with little actionability," says Abdo E. Kabarriti, MD, FACS.

"Portfolio rebalancing is a necessary part of an effective investment strategy, and a routine check of your investments should be completed regularly to ensure they have not drifted too far from their intended percentage targets," writes Jeff Witz, CFP.

The disease-free survival and distant metastasis-free survival benefits observed with adjuvant pembrolizumab in the intent-to-treat population of patients with clear cell renal cell carcinoma extended across all subgroups defined by disease risk, tumor stage, and lymph node involvement.

“A major finding from our study is how multiple sociodemographic, lifestyle, and medical factors influence testosterone levels in men," says Bu B. Yeap, MBBS, FRACP, PhD.

“Responsible application of AI is crucial, especially when the health and well being of people are at stake,” writes industry expert Manny Krakaris.
The authors suggest that metastasis-free survival may serve as a more suitable end point for future clinical trials of radiation therapy in localized prostate cancer.

"The purpose of the study is to compare the safety and efficacy of lutetium PSMA I&T vs standard of care hormone therapy in patients with metastatic castration-resistant prostate cancer," says Jason M. Hafron, MD.
Aquablation is a “minimally invasive procedure [that] is safe with long-term durability and a low-side effect profile,” said urologist Dennis Bentley, MD.

Erdafitinib previously received an accelerated approved for the treatment of adult patients with FGFR2/3-positive, previously-treated locally advanced or metastatic urothelial carcinoma.

"Saving and investing a modest amount regularly and early in your life can have a huge impact on your ability to reach your financial goals," writes Jeff Witz, CFP.
About 30% of patients received a diagnosis of ED and prediabetes or type 2 diabetes on the same day.

The UK REIMAGINE study suggests that MRI screening can not only bolster PSA screening, but also has the potential to be the central component of a national prostate cancer screening program.

Prostate cancer survivor Larry Ferguson, DMD, participated in a clinical trial at MUSC Hollings Cancer Center.

The BioProtect Balloon Implant System provides protection to the rectum during radiation therapy for prostate cancer.

In this episode, Janelle Bunce, PA-C, highlights the role that advanced practice providers can play in filling the gaps created by the growing workforce shortages in urology.

"There is nothing in the data to suggest any widespread abuse, improper inducements, or impropriety in the specialty. Nevertheless, urologists should be aware that this is an area of continued scrutiny by Congress and watchdogs," writes Robert A. Dowling, MD.

Cohort 3 of the SECuRE trial is exploring the safety and efficacy of a single-dose administration of 12GBq 67Cu SAR-bisPSMA in patients with metastatic castrate-resistant prostate cancer.

"I'm very pleased that this is now in the armamentarium for us," says Wayne G. Brisbane, MD.
The signatures were derived from the Decipher Genomics Resource for Intelligent Discovery (GRID) database in a retrospective analysis of the phase 2 STREAM study.

"These results show urologists that in this setting, pregabalin shouldn’t be routinely used," says Geoffrey H. Rosen, MD.

Benjamin N. Breyer, MD, is a urologic surgeon who is internationally known for performing complex urethral and penile reconstruction for urethra stricture and cosmetic disfigurement, male incontinence, male fistula, and surgery for erectile dysfunction.
The approval of the olaparib/abiraterone regimen was based on an exploratory subgroup analysis of the phase 3 PROpel trial consisting of the 85 patients in the study with BRCA-positive metastatic castration-resistant prostate cancer.

"We found that urology practices increasingly using mpMRI, and tissue-based genomics to a lesser extent, are more likely to treat men who have a high risk of non-cancer mortality. These men have the least to gain from treatment and are likely being overtreated,” says Kassem S. Faraj, MD, MS.

The application is specifically for use of enzalutamide in patients with nonmetastatic hormone-sensitive prostate cancer with high-risk biochemical recurrence and is supported by data from the phase 3 EMBARK trial.

"Our study raises concerns that some insurance plan policies are denying an effective, research-proven therapy, for a very common condition for which treatment is considered medically necessary," says Mohit Khera, MD, MBA, MPH.

The FDA's decision was based on results from the OASIS trial, in which the Revi tibial neurostimulator device demonstrated significant efficacy for treating urge urinary incontinence in women.

In predicting PCSM at 10 years, the area under the curve with the P-score was 0.93, compared with 0.81 with D’Amico and 0.88 with CAPRA.