
The novel agent ABSK061 approached a response rate of 40% in patients with advanced solid tumors harboring FGFR2/3 alterations.

The novel agent ABSK061 approached a response rate of 40% in patients with advanced solid tumors harboring FGFR2/3 alterations.

The combination is approved for the treatment of patients with metastatic castration-resistant prostate cancer, regardless of mutation status.

“Radiation therapy continues to expand its role, especially if you start talking about [patients with] the ultra high–risk [prostate cancer] or node-positive [disease] and beyond,” says Daniel Spratt, MD.

The Invitae Common Hereditary Cancers Panel utilizes DNA taken from a blood sample to identify the presence of variants in 47 genes linked to an increased risk of developing certain cancers.

Flotufolastat F 18 is now included in the NCCN prostate cancer guideline recommendations for all the same categories as the other PSMA-PET imaging agents approved by the FDA.
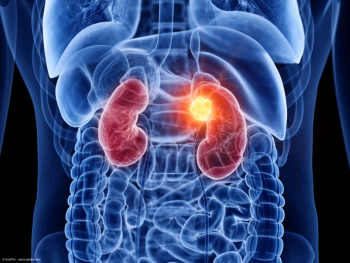
Investigations of treatment regimens featuring antibody-drug conjugates or radiotherapeutics are part of the ongoing wave of research in renal cell carcinoma, according to Rana R. McKay, MD.

The approval of flotufolastat F 18 (formerly 18F-rhPSMA-7.3) in prostate cancer is based on findings from the phase 3 SPOTLIGHT and LIGHTHOUSE trials.
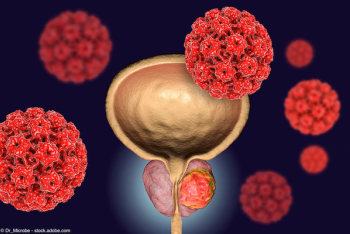
Data from the retrospective DEAR study linked darolutamide to lower rates of discontinuation and progression to metastatic disease vs enzalutamide and apalutamide in patients with nonmetastatic castration-resistant prostate cancer.

Data showed that treatment with talazoparib plus enzalutamide resulted in a 37% reduction in the risk of progression or death as assessed by blinded independent central review.

The application is supported by findings from a phase 1 trial and two phase 3 trials, SPOTLIGHT and LIGHTHOUSE.
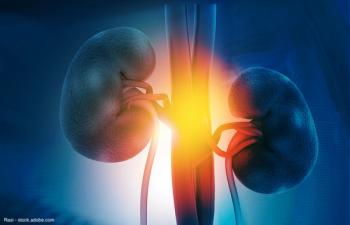
Data showed that nivolumab followed by nivolumab/ipilimumab resulted in an overall response rate of 34%.
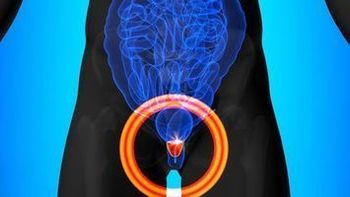
“These findings from the SPOTLIGHT study showed high inter-reader and intra-reader agreement for interpretation of 18F-rhPSMA-7.3 PET/CT scans in the setting of biochemical recurrence,” said Phillip Kuo, MD, PhD.
Compared with baseline conventional imaging, PET imaging with 18F-rhPSMA-7.3 frequently led to post-scan disease upstaging in men with prostate cancer recurrence, according to the latest data from the phase 3 SPOTLIGHT trial.

According to Dreicer, standard treatment in this setting for most patients should include androgen deprivation therapy (ADT) in combination with 1 or 2 other agents vs ADT therapy alone.
The addition of lenvatinib to pembrolizumab did not improve overall survival in patients with advanced urothelial carcinoma.

Published: July 26th 2023 | Updated:

Published: March 31st 2024 | Updated: