
The study is being conducted as part of the landmark ProtecT trial.

The study is being conducted as part of the landmark ProtecT trial.

The code, J9282, went into effect on January 1, 2026.

According to the authors, these findings support the early use of genomic testing to guide treatment decisions.

A recap of the FDA submissions and regulatory decisions in urology from December 2025.

As the year comes to a close, we revisit some of this year’s top content in female urology.

As the year comes to a close, we revisit some of this year’s top content in men's health.

As the year comes to a close, we revisit some of this year’s top content on bladder cancer.
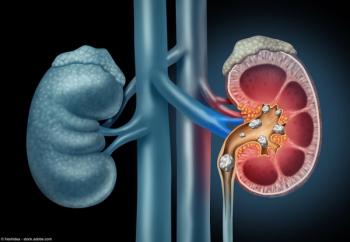
As the year comes to a close, we revisit some of this year’s top content on prostate cancer.

As the year comes to a close, we revisit some of this year’s top content on benign prostatic hyperplasia.

As the year comes to a close, we revisit some of this year’s top content on genomic testing.

As the year comes to a close, we revisit some of the top Urology Times podcast episodes from 2025.

As the year comes to a close, we revisit some of this year’s top content on prostate cancer.

The application is supported by results from the phase 3 PIVOT-PO trial.

The PROSTOX ultra test identifies men with localized prostate cancer who are at a higher risk of developing late GU toxicity from radiation, helping to inform treatment decisions.

The approval is supported by data from the TRITON3 trial.

Patients with GG1 disease and a high genomic classifier score had a significantly higher risk of disease progression.

The combination yielded significant improvements in event-free survival, overall survival, and pathologic complete response rates vs neoadjuvant chemotherapy.

The combination demonstrated a disease-specific survival rate of 98.7% at 12 months and 96.0% at 36 months.

The safety and efficacy of the Revi System were established in the pivotal OASIS trial.

With this approval, flibanserin becomes the first and only treatment of its kind for women younger than 65 years.

The approval is supported by data from the phase 3 AMPLITUDE trial.

In a phase 3 trial, zoliflodacin demonstrated non-inferior efficacy compared with ceftriaxone plus azithromycin.

Nogapendekin alfa inbakicept was approved in combination with BCG in the US in April 2024.

The approval is supported by data from the phase 3 EAGLE-1 trial.

In a panel meeting convened by the FDA, experts urged the agency to revise the current classification and indication for testosterone replacement therapy in men.

The approval is supported by results from the randomized ProVIDE study.

The pilot study plans to enroll patients with locally recurrent prostate cancer across clinical trial sites in the US.

The FDA has selected detalimogene voraplasmid to participate in the Chemistry, Manufacturing, and Controls Development and Readiness Pilot Program.

The phase 1 study will assess the safety and feasibility of SPECT/CT imaging with [111In]In-ART-101.

DFS rate was 85.3% at 6 months (95% CI, 71.6-92.7), 74.3% at 12 months (95% CI, 59.2-84.6), and 69.2% at 18 months (95% CI, 53.4-80.6).