
Dr. Primo Lara, Jr., executive associate dean for Cancer Programs, School of Medicine, at UC Davis Comprehensive Cancer Center, highlights the need for personalized medicine using multidisciplinary care for patients with renal cell carcinoma.

Dr. Primo Lara, Jr., executive associate dean for Cancer Programs, School of Medicine, at UC Davis Comprehensive Cancer Center, highlights the need for personalized medicine using multidisciplinary care for patients with renal cell carcinoma.

Scott T. Tagawa, MD, highlights pivotal research and next steps with the alpha particle–emitting radioactive therapeutic agent radium-223 in prostate cancer.

“As we suspected, the more work interfered with their freedom to use the restroom, the fewer times they urinated during the work day,” says W. Stuart Reynolds, MD, MPH, FACS.

Jatenzo is approved by the FDA as a testosterone replacement therapy for men with certain forms of hypogonadism.
Dr. Leonard G. Gomella discusses emerging biomarkers and the use of MRI for the early detection of prostate cancer.

Michael E. Hurwitz, MD, PhD, discusses how immune checkpoint inhibitors have transformed the first-line setting in advanced renal cell carcinoma.
“For now, the UMPIRE [study] protocol will continue to withhold prophylaxis in the newborn population,” says investigator M. Chad Wallis, MD, FACS.

Watch the on-demand video of Urology Times' March 2021 Around the Practice.

“The way I think about this test now is… more as a dichotomous score,” says Eric A. Klein, MD.



“It’s really fascinating how the field is expanding,” Klein says.
“The take-home message is that for the appropriate patient, each of [these] technologies works well,” says Richard K. Lee, MD, MBA.

“It’s an extremely useful tool,” says Jonathan D. Tward, MD, PhD, of the Prolaris test.
Investigators observed a decline in the number of men obtaining repeat postvasectomy semen analyses.

The book incorporates insights from 45 experts across 18 specialties.

A search of a legal database found 42 cases related to cystectomy over a 30-year period.
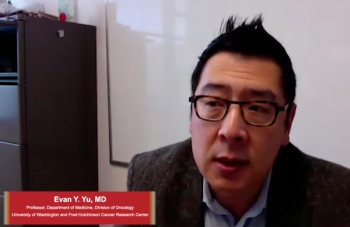
Yu discusses the background of PSMA-PET imaging, current and emerging PSMA imaging tools/therapeutics, and the future of the novel technique in prostate cancer.

“The role of genomic testing in bladder is expanding almost every year,” says Faltas.

In the phase 2 SWOG 1500 study, cabozantinib (Cabometyx) significantly improved progression-free survival versus sunitinib (Sutent) in patients with metastatic papillary renal cell carcinoma.

“Very, very few patients actually had any counseling documented or any medications ordered that would help with smoking cessation,” says Richard S. Matulewicz, MD.
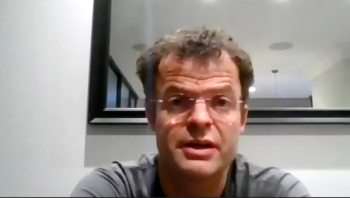
Treatment following progression on chemotherapy and an immune checkpoint inhibitor remains an unmet medical need.

Shaakir Hasan, DO, discusses socioeconomic characteristics associated with the diagnosis and management of bladder cancer.

O’Leary discusses safety related to instillation of the Penuma penile enhancement implant.

Darolutamide is currently approved for the treatment of patients with nonmetastatic castration-resistant prostate cancer.

The immunotherapy/TKI combination significantly improved overall survival versus sunitinib.

“I find [the Prolaris test] to be a very helpful clinical tool,” says Jonathan D. Tward, MD, PhD.

Hear from key opinion leaders in the urology space as they discuss 2 complex cases across the multidisciplinary approach for an incidentally discovered adrenal mass and localized, high-risk prostate cancer with oligometastasis at diagnosis.

