
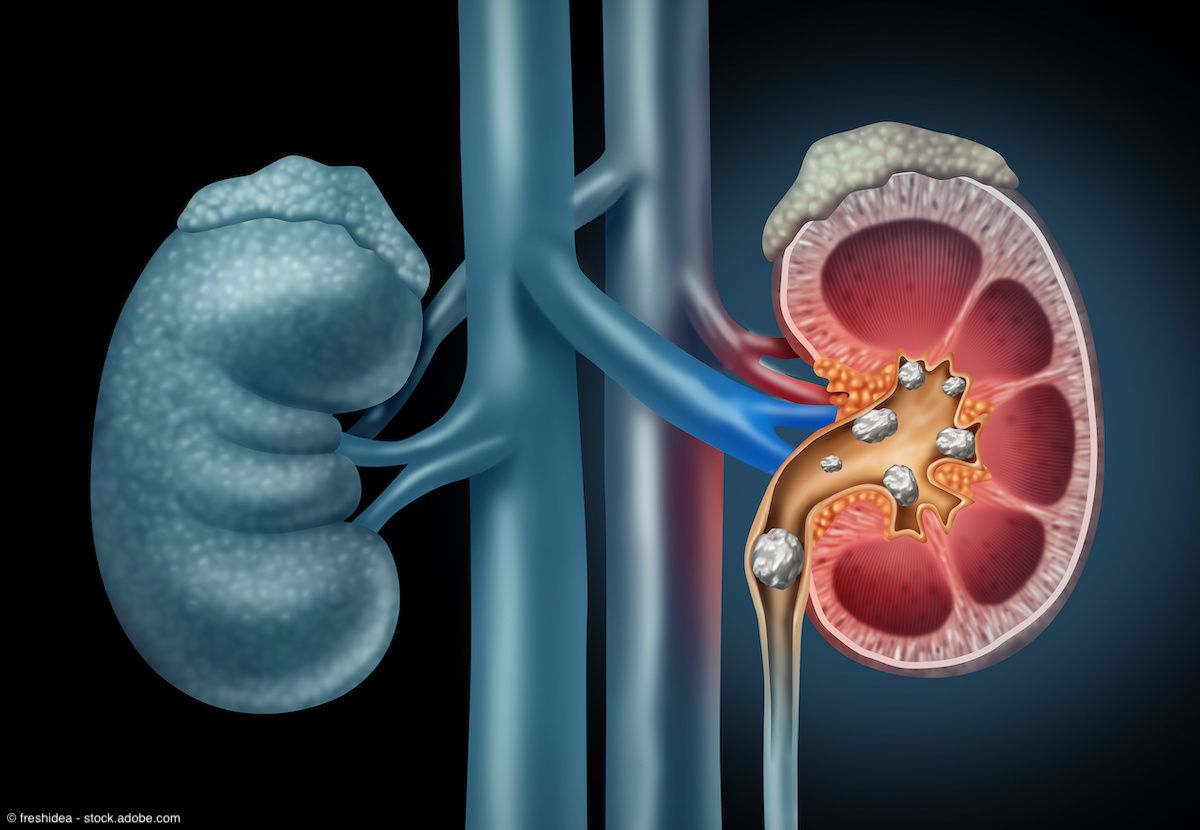
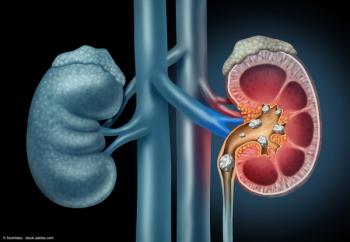
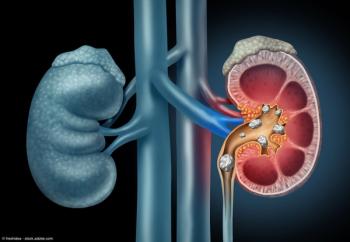
Kidney Stones
Latest News
Latest Videos

CME Content
More News

This announcement follows the successful launch of generic tiopronin delayed-release tablets last year.
The overall prevalence of kidney stones remained stable, with a notable increase observed among women.

“We might need to do a better job in general about education of the importance of follow-up,” says Christina B. Ching, MD.

“Interestingly, the patients that were more likely to follow-up were those patients that were seen and established within our urology clinic,” says Christina B. Ching, MD.

“We felt, not only for the study, but for providing good care, that it was important to try and have these patients follow-up and see if we could identify factors that might impact their follow-up,” says Christina Ching, MD.

A recap of the FDA submissions and regulatory decisions in urology from February 2025.
YOLT-203 was granted an Orphan Drug Designation and a Rare Pediatric Disease Designation in September 2024.

A phase 1/2 trial of ABO-101 is expected to launch in the first half of 2025.

As the year comes to a close, we revisit some of this year’s top content on kidney stone management.

The SURE procedure also demonstrated significantly better outcomes on the study’s secondary end points of stone clearance and residual stone volume.

The redePHine trial is expected to launch in the first half of 2025.

"The Stone Clear device provides patients with a non-invasive option to reduce their residual fragment stone burden in the clinic environment while being fully awake," says James E. Lingeman, MD.

“So, we have all of this new technology that's about a year old, making surgery a lot safer for patients, making things more efficient, and [giving us] the ability to see stone removal happening from a perspective where we can visualize stone clearance in a way that we haven't been able to before,” says Marcelino E. Rivera, MD.

"[One] of the things that makes the technology really interesting, in my mind, for CVAC, is that it's not just a suction device," says Thomas Chi, MD, MBA.

"From a revenue, profit and loss perspective, actually, in many instances, they anticipate that physicians may actually make more money using the second-generation CVAC device compared to traditional ureteroscopy, depending on your payer mix and your practice," says Thomas Chi, MD, MBA.
Thomas Chi, MD, MBA, discusses advantages to the CVAC System.

"What we demonstrated was that at 30-day and 90-day interval outcomes, the patients actually had equivalent safety profiles compared to traditional ureteroscopy with basketing," says Thomas Chi, MD, MBA.

YOLT-203 is currently being assessed in the early phase 1 YOLT-203-101 trial.

"I think we could explore risk adjustment for surgeon reimbursement as a way of incentivizing providers and compensating them fairly for taking care of these more complex patients," says Victoria S. Edmonds, MD.

"For more rural practices, for ureteroscopy, they also received lower reimbursement than urban practices," says Victoria S. Edmonds, MD.
In total, 8 of 40 patients who underwent ultrasonic propulsion experienced relapse, compared with 21 of 42 patients who underwent observation.

“What this means for patients is that thiazides remain an important option in the toolkit for preventing kidney stone recurrence,” says Ryan S. Hsi, MD, FACS.
In total, the trial plans to enroll 7 patients with PH1 through a single center in China.

“The great news is that we have now completed recruitment. We actually recruited more than 1700 patients,” says Kari A.O. Tikkinen, MD, PhD.

Any stone fragmentation was achieved in 88% of patients.















