
Prostate Cancer
Latest News
Latest Videos

CME Content
More News

"This study provides a comprehensive review of the role of natural products as complementary treatments for prostate cancer," says Channing J. Paller, MD.

Overall, 39% of those who received a university-formatted report and 56% of those who received a VA-formatted report were able to identify that they had prostate cancer.
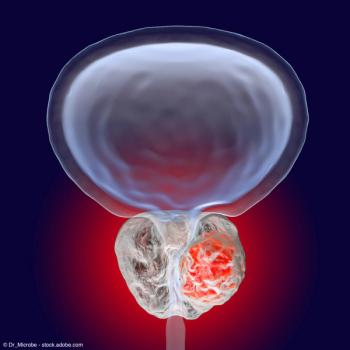
At 25 weeks, FACT-P total score remained comparable between the arms.

"If you can do this surgery well, patients actually go back to living a normal life, and they're immensely, immensely happy," says Jaspreet S. Sandhu, MD.

"We are trying to develop epigenetic biomarkers that can predict patients that are starting to progress to neuroendocrine prostate cancer," says Jindan Yu, MD, PhD.

The Mona Lisa 2.0 platform has been previously approved in the US, Australia, and Singapore.

The PDUFA target action date has been set for August 29, 2025.

“Another aspect is how to make this more and more complex diagnostic chain for prostate cancer work in practice, because we now have so [many] more tools than we had a decade ago,” says Tobias Nordström, MD, PhD.

Following an initial dose of VIR-5500 of 120 µg/kg or higher, all patients achieved a PSA reduction.

“The key take home message of that study was that having assumptions about basic medical terminology that we think are simple may actually leave patients confused,” says Vikram M. Narayan, MD.

“If there's enough radiation, then the cells can't repair themselves and die because of the radiation,” says Scott T. Tagawa, MD, MS, FACP, FASCO.

The application is supported by data from the pivotal phase 3 ARANOTE trial.

Catch up on all the notable drug and device approvals in urology over the past year.

As the year comes to a close, we revisit some of this year’s top video content from Urology Times.
As the year comes to a close, we revisit some of this year’s top content on prostate cancer.

"There's a number of emerging modalities in the primary focal ablation space that are in trial at this time," says Kara L. Watts, MD.

The alert was issued to clarify that the devices should not be used for suction and irrigation.
“Our findings highlight the coordinated interplay between GCN2 and p53 regulation during nutrient stress and provide insight into how they could be targeted in developing new therapeutic strategies for [prostate cancer]," write the authors.

Pluvicto was approved by Health Canada in August 2022.

From baseline to 1 year, the Ki-67 index decreased in the dietary intervention cohort by 15% and increased in the control cohort by 24%.

"We found that with a 5-year Kaplan-Meier survival analysis that patients with varicocele had worse outcomes than patients without varicocele," says Muhammed A. Moukhtar Hammad, MBBCh.

“If approved, this approach has the potential to transform the treatment paradigm in prostate cancer, offering patients with localized disease an effective treatment option that may reduce the risk of disease recurrence," says Glen Gejerman, MD, MBA.

Specifically, 90% of patients in the HIFU arm vs 86% of patients in the radical prostatectomy arm were free from subsequent definitive treatment at 30 months.

The clearance is supported by data from the PRESERVE trial, which evaluated the safety and effectiveness of the NanoKnife System in patients with intermediate-risk prostate cancer.

PT217 is currently under investigation in the phase 1/2 SKYBRIDGE trial in patients with neuroendocrine cancers.
















