
“At 60 months, nadofaragene allowed bladder preservation in nearly half of the patients in the CIS cohort and two-thirds of patients in the Ta/T1 cohort,” said Vikram Narayan, MD.

“At 60 months, nadofaragene allowed bladder preservation in nearly half of the patients in the CIS cohort and two-thirds of patients in the Ta/T1 cohort,” said Vikram Narayan, MD.
A post hoc analysis of the phase 3 ATLAS trial showed that UGN-102 with or without TURBT induced meaningful disease-free survival and duration of response in patients with non–muscle-invasive bladder cancer.
"[Nivolumab] plus [gemcitabine-cisplatin] showed significant and clinically meaningful benefit in OS, PFS, and response over [gemcitabine-cisplatin] alone," said Guru Sonpavde, MD.
"[The] NURE-Combo trial provided insights into the potential value of expanding chemotherapy combinations with immune checkpoint [inhibitors] in muscle-invasive disease," said Chiara Mercinelli, MD.
EG-70 is an experimental intravesical immunotherapy that does not involve viruses or integration. It aims to eliminate tumors by triggering coordinated innate and adaptive immune reactions specifically within the bladder.

Treatment with the Zenflow Spring System, a minimally invasive surgical therapy for the treatment of benign prostatic hyperplasia, does not cause negative sexual side effects.
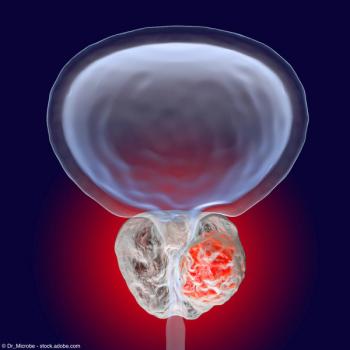
Lutetium Lu 177 vipivotide tetraxetan improved rPFS vs switching to abiraterone acetate or enzalutamide in patients with taxane-naïve mCRPC who had progressed on prior abiraterone or enzalutamide. Outcomes were better, however, in patients who initially received abiraterone.

More patients with nonmetastatic castration-sensitive prostate cancer reached an undetectable PSA level if they received enzalutamide, as a single agent or combined with leuprolide, vs if they received leuprolide alone.

The addition of 18F-DCFPYL PSMA-PET imaging to multi-parametric MRI was associated with an improvement in the detection of clinically significant prostate cancer in men on active surveillance.

Steerable ureteroscopic renal evacuation using the CVAC Aspiration System demonstrated noninferiority vs standard ureteroscopy for kidney stone removal, according to findings from the prospective, randomized ASPIRE trial.
Treatment with the intravesical gemcitabine delivery system TAR-200 led to complete responses in over 80% of patients with BCG-unresponsive, high-risk non–muscle-invasive bladder cancer, according to the latest results from the phase 2b SUNRISE-1 trial.
Extended lymph node dissection during radical cystectomy offered no additional overall survival or disease-free survival benefit vs standard lymph node dissection.

The UroActive artificial urinary sphincter devices were successfully implanted and activated in all patients.
“These findings support the use of 18F-PSMA-1007 PET/CT in the preoperative workflow of intermediate-and high-risk tumors,” said Nikhile Mookerji, MD.

Treatment with apalutamide and androgen deprivation therapy led to a 2-year biochemical recurrence-free survival rate of 100% in patients with high-risk, post-prostatectomy prostate cancer.

Qmax was also significantly improved, with a baseline of 7.7 mL/sec compared with 13.2 mL/sec at 4 years.
The novel oncolytic immunotherapy cretostimogene grenadenorepvec induced complete responses in three-fourths of patients with BCG-unresponsive non–muscle-invasive bladder cancer with carcinoma in situ, according to findings from the phase 3 BOND-003 trial.

In the world of health care, factors such as remote care, complex IT environments, and connected health care devices are creating new avenues for cybercriminals to target.

Rohan Garje, MD, discusses precision medicine in metastatic prostate cancer, including genomic testing, targeted therapies, and theragnostics.

"[This] shows a different approach for detecting circulating tumor DNA in bladder cancer patients, and it opens up the possibility for other biorepositories that have serum samples to also enter the ctDNA research environment," says Richard T. Bryan, MBChB, PhD, MRCS, FacadTM.

Investigators will begin setting up the TRANSFORM trial in spring 2024, with study recruitment set to begin later in the year

The test, which was launched in Canada in September 2023, is available through Nanostics’ accredited laboratory in Edmonton, Alberta.

"We felt that it was important to again address this topic because we have received numerous questions regarding the correct use of this code and we have had some experience using the code now that it is active and been able to observe some of the initial payment processing by the payer," write Jonathan Rubenstein, MD, and Mark Painter.

"Results from these trials show that giving physicians an alert informing them of their patient’s actual risk for antibiotic resistance can help them choose the best antibiotic and reduce extended-spectrum antibiotic use,” says Shruti Gohil, MD, MPH.

All patients have been successfully dosed in the first cohort of patients, and the Safety Review Committee has granted approval for progression of the study to the second dose level of INKmune.

"The results showed the contemporary Aquablation procedural safety in Japanese men is exceptional in a broad range of prostate sizes along with significant symptom reduction," says Nobuyuki Hinata, MD.

"This was a very special moment, delivering the news to this patient that his cancer is now undetectable following the treatment with 2 doses of 8 GBq of 67Cu-SAR-bisPSMA," says Luke Nordquist, MD, FACP.

A late phase 3 trial evaluating the agent is underway at several clinics in Europe.
“This clinical study has the potential to demonstrate the value of Chimeric Antigen Receptor (CAR) T cell therapy in solid cancers such as kidney cancer with a high unmet medical need,” said Abla Creasey, PhD.
The first patient was treated with TLX250-CDx at the Olivia Newton-John Cancer Wellness Centre at Austin Health in Melbourne, Australia,