Prostate Cancer
Latest News

Latest Videos

CME Content
More News
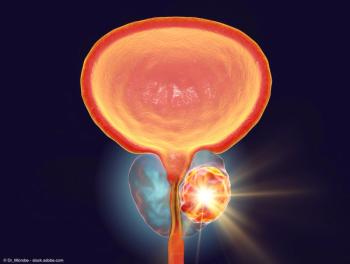
The Focal One high intensity focused ultrasound system uses precision sound waves to destroy prostate tumor tissue and spare healthy tissue, reducing the risk of side effects that would lower a patient's quality of life.

“I was of course flattered when Dr. Walsh and Janet Worthington approached me to take the lead on editing and writing this fifth version of the book,” says Edward M. Schaeffer, MD, PhD.
"FPSA is an important, inexpensive, and readily available test in our risk-stratification toolbox," writes Badar M. Mian, MD.

Joshi Alumkal, MD, highlights findings from the phase 3 ARASENS trial that led to the FDA approval of darolutamide for use in combination with docetaxel for the treatment of patients with metastatic hormone-sensitive prostate cancer.

In the third article of this series, Amar U. Kishan, MD, provides clinical insights on the use of perirectal spacers for patients who receive hypofractionated radiation or SBRT.

“About 25% of men didn't even want to know that they had cancer,” says Daniel E. Spratt, MD.
“What's so great about the ArteraAI platform is the simplicity in which this test is performed,” says Rana R. McKay, MD.

"This could make a huge impact for patients with limited resources,” says Benjamin Pockros, MD, MBA.

"Encouraging our patients to adopt a healthy, plant-based diet is a great way to simultaneously promote both prostate and overall health," writes Stacy Loeb, MD, MSc.

Shared insight into FDA-approved PSMA PET imaging tracers, highlighting similarities and differences between gallium 68 and fluorine 18 compounds.

Naveen Kella, MD, and Dr. Shadi Esfahani, MD, MPH, delve into patient selection for PSMA PET CT scans, discussing indications spanning initial diagnosis and recurrence, as well as institution guidelines.

Men who had a dorsal testosterone/peripheral testosterone ratio greater than 2 were found to have higher rates of PSA persistence compared with patients who had a similar ratio.
The published findings show the viability of using a transperineal ultrasound system as a means to monitor intra-fractional prostate motion for prostate stereotactic body radiotherapy.

Experts explore PSMA's significance in prostate cancer imaging, covering its expression, limitations, and transformative role in detecting even low PSA recurrences with PSMA-PET technology.

In this introductory dialogue, Naveen Kella, MD, and Dr. Shadi Esfahani, MD, MPH, discuss PSMA-PET's transformative role alongside conventional techniques in prostate cancer imaging.

The authors suggest that metastasis-free survival may serve as a more suitable end point for future clinical trials of radiation therapy in localized prostate cancer.

The UK REIMAGINE study suggests that MRI screening can not only bolster PSA screening, but also has the potential to be the central component of a national prostate cancer screening program.

Prostate cancer survivor Larry Ferguson, DMD, participated in a clinical trial at MUSC Hollings Cancer Center.

The BioProtect Balloon Implant System provides protection to the rectum during radiation therapy for prostate cancer.

In the third installment of this series, Amar U. Kishan, MD, discusses clinical factors to consider in the use of perirectal spacing for patients receiving hypofractionation or SBRT, and outlines unmet needs.

"I'm very pleased that this is now in the armamentarium for us," says Wayne G. Brisbane, MD.
The signatures were derived from the Decipher Genomics Resource for Intelligent Discovery (GRID) database in a retrospective analysis of the phase 2 STREAM study.

Closing out their discussion on how treatment and management of advanced prostate cancer affects their clinical practice, expert urologists consider how the therapeutic landscape continues to evolve and look toward future evolutions in care.
Expert panelists share their perspectives on recent challenges with staffing and turnover in their urology practice and consider how these particular barriers may be overcome, including recruiting and hiring additional advanced practice providers.

The application is specifically for use of enzalutamide in patients with nonmetastatic hormone-sensitive prostate cancer with high-risk biochemical recurrence and is supported by data from the phase 3 EMBARK trial.