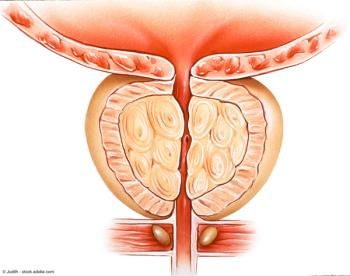
“Our results demonstrated that treatment with [the] Optilume BPH procedure resulted in significant symptom relief while still preserving erectile and ejaculatory function,” said Olivia Copelan, MD.
Benjamin P. Saylor is associate editor of Urology Times, an Advanstar Communications publication.

“Our results demonstrated that treatment with [the] Optilume BPH procedure resulted in significant symptom relief while still preserving erectile and ejaculatory function,” said Olivia Copelan, MD.
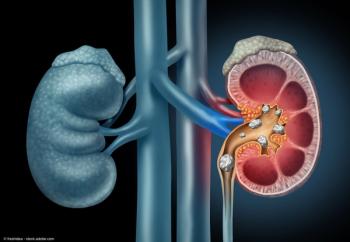
“We found that the SAPS is associated with higher intrarenal pressure and flow rate when compared to automated pumps," said Lucas B. Vergamini, MD.

Patients receiving PUL experienced improvements of 8.6 and (39.1%) and 10 (46.8%) in IPSS total score.
The investigators found that the propulsion group had a 70% lower risk of relapse than the control group (HR 0.30, 95% CI: 0.13-0.68).
"[Nivolumab] plus [gemcitabine-cisplatin] showed significant and clinically meaningful benefit in OS, PFS, and response over [gemcitabine-cisplatin] alone," said Guru Sonpavde, MD.
"[The] NURE-Combo trial provided insights into the potential value of expanding chemotherapy combinations with immune checkpoint [inhibitors] in muscle-invasive disease," said Chiara Mercinelli, MD.

Qmax was also significantly improved, with a baseline of 7.7 mL/sec compared with 13.2 mL/sec at 4 years.

The test, which was launched in Canada in September 2023, is available through Nanostics’ accredited laboratory in Edmonton, Alberta.

A late phase 3 trial evaluating the agent is underway at several clinics in Europe.
“This clinical study has the potential to demonstrate the value of Chimeric Antigen Receptor (CAR) T cell therapy in solid cancers such as kidney cancer with a high unmet medical need,” said Abla Creasey, PhD.

Petros also announced that it has received positive feedback from the FDA after the agency’s informal review of Petros’ technology component for self-selection.

Regarding safety, the investigators did not observe any grade 3 or 4 adverse events during the study.
The investigators reported that “the risk of recurrence was significantly lower following BLC (HR=0.67, 95% CI: 0.51-0.89) than WLC.”

The investigators reported that pCR was significantly higher in the neoadjuvant chemotherapy + RNU group vs RNU alone, with an OR of 2.49 (95% CI: 1.75 – 3.54, P < .001).

At baseline, 16 patients had an IIEF-EF score of 11-21. These patients reported significant improvement in IIEF-EF score at Times 2 and 4, according to the authors.

"An educated patient is one that's always going to want to be more involved in trying to make themselves better; they're going to be an empowered patient,” says Thomas J. Mueller, MD.

“No doubt about it, this is unequivocally a positive trial by the rPFS criteria. Quite positive,” says A. Oliver Sartor, MD.
“Belzutifan plus cabozantinib continue to show durable anti-tumor activity in patients with clear cell RCC who are treatment naïve or previously treated,” said Toni K. Choueiri, MD.

An exclusive Urology Times survey measured US-based urologists’ uptake, access to, and indications for utilizing prostate-specific membrane antigen-targeting agents.

The FDA's decision was based on results from the OASIS trial, in which the Revi tibial neurostimulator device demonstrated significant efficacy for treating urge urinary incontinence in women.
"The addition of an immune checkpoint inhibitor after tri- or tetra-modality therapy might improve long-term outcomes for patients with bladder cancer,” said Maria De Santis, MD.

"In EV-103 dose escalation/cohort A, the safety profile of the combination was manageable, with no new safety concerns observed at nearly 4 years of follow-up," said Shilpa Gupta, MD.

The proportion of patients who discontinued initial ARI treatment was 30.4% for darolutamide compared with 40.8% for enzalutamide and 46.0% for apalutamide.

"PRP does not seem to be effective in treating men with erectile dysfunction, at least as a monotherapy,” said Ranjith Ramasamy, MD.

“We looked at safety issues within 12 months after the implantation, and they were minor—some pain problems, some wound-healing problems, but no serious adverse events,” said John Heesakkers, MD, PhD.

“What we found is that hypertension is an independent risk factor for worsened survival outcomes and mortality outcomes across the board on multivariable and Kaplan Meier analysis,” said Sohail Dhanji, MD.

Investigators retrospectively studied patients with Peyronie disease and diabetes mellitus between 2007 and 2022.

"Patients with a prior BPH procedure were on average older than our HoLEP controls; however, there were no other differences in preoperative patient characteristics or postoperative functional outcomes," said Nicholas S. Dean, MD.

Clinical progression-free survival following the first cycle of radioligand therapy was 6.3 months, and overall survival following the first cycle was 21.4 months.
In cohort B of the phase 2 Keynote-057 trial, pembrolizumab led to antitumor activity in patients with BCG-unresponsive, papillary high-risk non–muscle-invasive bladder cancer.