
Here’s a look back at notable news between July and September 2025.
Benjamin P. Saylor is associate editor of Urology Times, an Advanstar Communications publication.

Here’s a look back at notable news between July and September 2025.

We recap notable headlines from last month in the benign urology space.

A comprehensive guide to the key regulatory decisions and conferences slated for the last few months of the year.

Take a closer look at what urologists can expect from the LBAs that will headline ESMO 2025.
The biomarker panel demonstrated the ability to detect prostate cancer even in men with prostate-specific antigen levels in the normal range.

We recap notable headlines from last month in the benign urology space.

The ArteraAI test is now the first and only AI-powered tool to be authorized to prognosticate long-term outcomes in patients with localized prostate cancer.

“This designation is a powerful validation of our software's potential to transform how we treat cancer,” said Andre Esteva, CEO of Artera.

According to the news release, detailed OS data from EMBARK are set to be presented at an upcoming medical conference.

The investigational treatment is being evaluated in phase 1 clinical trials for solid tumors.

Among the content is additional information on types of urinary incontinence, ways to control urgency, and frequently asked questions about a healthy bladder.

According to the company, the MHRA approval represents the first marketing approval outside the US for NAI.

Eligible patients will receive UGN-103 75 mg intravesically once per week for 6 weeks.

For the trial, a biomarker will be utilized to aid in patient selection for the treatment.

Here’s a look back at notable news between April and June 2025.

A comprehensive guide to the key regulatory decisions and conferences slated for Q3 of 2025.
The approach was associated with a 30% decrease in PSMA uptake by the salivary glands without compromising the treatment’s efficacy.

ORR in the zanzalintinib plus nivolumab arm was 63% (95% CI: 46-77) vs 40% (95% CI: 25-57) in the zanzalintinib plus nivolumab/relatlimab arm.

In 2015, 1245 patients had prostate cancer and had also initiated a GLP-1 or GIP/GLP-1 receptor agonist compared with 69,808 patients in 2024.

Treatment with darolutamide was found to extend median time to deterioration of FACT-P total score by 5.1 months vs placebo

“This was a positive study, resulting in a statistically significant improvement in the primary end point of rPFS in the intent-to-treat population," said Rana R. McKay, MD, FASCO.

Clinical benefit rate was observed 5 (27%) patients in cohort 1, 7 (27%) patients in cohort 2, and 2 (11%) patients in cohort 3.

“As of April 2025, 616 patients have been randomized,” said Alicia Morgans, MD, MPH.

“Prior taxanes did not increase reported hematological adverse events during treatment with Ra-223,” the investigators reported.
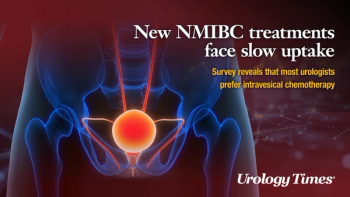
Results of an exclusive Urology Times survey point to urologists preferring intravesical chemotherapy for treating BCG-unresponsive NMIBC.

“On the intention-to-treat efficacy analysis...the median PFS was numerically higher for the paclitaxel arm," said Aditya Dhanawat, MBBS, MD, DM.
The findings also lend further credence to the muscle-invasive bladder cancer treatment regimen, investigators reported.

"The pre-specified thresholds for statistical significance were not met for the dual primary end points of overall survival in cisplatin-ineligible and PD-L1 positive patients," said Michiel Van der Heijden, MD, PhD.

"There was activity across all the groups from an EFS perspective," said Thomas B. Powles, MBBS, MRCP, MD.

Vitaly Margulis, MD, reported an overall response rate of 86.5%, and a complete response rate of 73%.