
The approval was based on data from the ROBUST I and ROBUST 3 clinical trials.

The approval was based on data from the ROBUST I and ROBUST 3 clinical trials.

The once-daily capsule combines 5 mg of tadalafil with 5 mg of finasteride.

Catch up on all the notable drug and device approvals in urology over the past year.
The FDA issued a complete response letter earlier this year regarding a biologics license application for the protein fusion drug in patients with BCG-unresponsive non–muscle invasive bladder cancer.

The PROPELLER trial is exploring the novel PSMA-PET imaging product 64Cu-SAR-bisPSMA in patients with treatment-naïve, histologically confirmed prostate cancer who are scheduled for radical prostatectomy.
Transperineal biopsy can be used to lower the risk of infectious complications even if antibiotic prophylaxis is not used.
Adding darolutamide to docetaxel and androgen deprivation therapy significantly improved overall survival in men with metastatic hormone-sensitive prostate cancer.
"We sought to conduct a National Cancer Database analysis evaluating the impact of the extent of lymphadenectomy on survival outcomes for patients presenting with intermediate- and high-risk prostate cancer,” said Furkan Dursun, MD.

A majority of women were able to return to sexual activity post-surgery without compromising oncological outcomes.

The RENOVA iStim tibial neuromodulation system is a wireless peripheral neurostimulator device that a clinician implants in the patient’s ankle with the patient under local anesthesia.

The approval was based on data from the phase 3 KEYNOTE-564 trial.
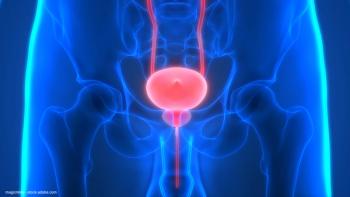
All efficacy-evaluable patients reached a complete response with the combination regimen.

Now that there are 2 FDA-approved PSMA-PET imaging agents, urologists and other clinicians are asking about the differences between them, as well as how PSMA-PET imaging compares to the previously approved next-generation imaging agent fluciclovine F 18.

At the 2021 LUGPA Annual Meeting, Steven Rowe, MD, PhD, discussed the paradigm-shifting emergence of PSMA-PET imaging in prostate cancer.
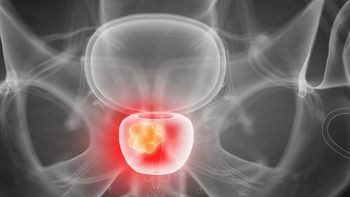
The SECURE trial is exploring the PSMA PET imaging product 64Cu-SAR-bisPSMA and the PSMA targeted therapy 67Cu-SAR-bisPSMA.
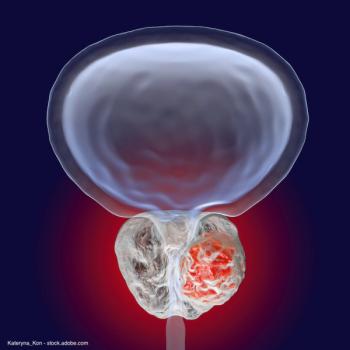
The procedure involves using MRI/ultrasound fusion imaging technology to direct focal ablation of prostate tissue using nanoparticle-directed laser ablation.

"In this cohort study, cabozantinib showed considerable intracranial activity and an acceptable safety profile in patients with RCC and brain metastases," the authors wrote.

The oncology provider will enable patient access to the PSMA-PET imaging agent piflufolastat F 18, which the FDA approved in May 2021 for identifying suspected metastasis or recurrence of prostate cancer.

"Combining mpMRI and 68Ga-PSMA PET/CT in a primary diagnostic setting could better identify where to target on biopsy, increasing the diagnostic yield, improving concordance with underlying tumor grade and therefore improving management recommendations," the authors wrote.

In the majority of patients, the study findings resulted in impactful changes to clinical management.

The nomogram was significantly prognostic of key clinical end points and achieved outcomes comparable to STAR-CAP and superior to other established risk-stratification tools.

“Additional Peyronie’s disease therapies are needed as current treatment modalities have limited efficacy and considerable side effects," the authors wrote.

Data from the phase 2/3 QUILT-3.032 trial showed that adding N-803 to BCG met the primary end point of disease-free survival in patients with BCG-unresponsive, high-grade non–muscle-invasive bladder cancer and papillary disease.

“Optilume exhibited a significant improvement in [the primary] objective outcome at 6 months and subjective outcomes at 1-year post treatment compared to standard of care,” said Justin Chee, MBBS, FRACS.

“If results of this study are compelling, we may have the opportunity to submit them to the FDA as part of an application for accelerated approval," said Roger Dansey, MD.
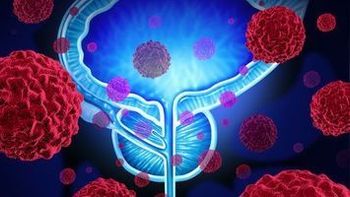
Early research has shown the capacity of PVSRIPO to activate a patient's immune system to help trigger a systemic antitumor response.

“The efficiency of the procedure and clear visual opening of the prostatic urethral channel makes the Zenflow [Spring System] an exciting new tool in the treatment for BPH,” said Michael D. Trotter, MD.

The significant treatment response and sustained durability observed in this trial indicate that UGN-102 has the potential to become a nonsurgical alternative for these chronically relapsing patients, who typically undergo repetitive surgeries,” said William C. Huang, MD.

The designation, which will expedite the regulatory review of LuPSMA in this setting, is primarily based on findings from the phase 3 VISION trial.
The phase 3 SPLASH trial is exploring the novel PSMA-targeted therapy PNT2002 in patients with metastatic castration-resistant prostate cancer and progressive disease.