
There was not a similar reduction in nocturnal voids among patients with a decrease in systolic blood pressure.

There was not a similar reduction in nocturnal voids among patients with a decrease in systolic blood pressure.
The PET radiopharmaceutical led to a shift in the management of nearly two-thirds of men with biochemical recurrence of prostate cancer.
The approval was primarily supported by data from the phase 3 EV-301 trial, in which enfortumab vedotin reduced the risk of death by 30% versus chemotherapy in patients with heavily pretreated locally advanced or metastatic urothelial carcinoma.

A decrease in urinary ATP levels following onabotulinumtoxinA (Botox) injections corresponded to clinical improvements in overactive bladder symptoms.
The results also showed a trend toward improved overall survival with the combination of the PARP inhibitor and novel hormonal agent; however, the data remain immature.

The OAB symptoms, known as COVID-19–associated Cystitis, include increased urinary urgency, frequency, nocturia, and pain.
Combining avelumab with standard neoadjuvant chemotherapy induced pathological complete responses in over half of patients with nonmetastatic muscle-invasive bladder cancer.

Paige Prostate AI-based software provides pathologists with a supplementary assessment of prostate biopsy images that identifies the area with the highest likelihood of harboring cancer.
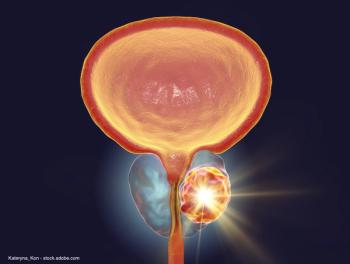
The results included data for 132 patients, all of whom had prior enzalutamide and/or abiraterone acetate.

"Clinical benefit was observed regardless of homologous recombination repair gene mutational status," the study authors wrote.

Multiple PSMA-PET imaging agents are approved by the FDA for use in prostate cancer.

The guideline update is supported by findings from the pivotal RTOG 96-01 trial.

Among the key findings was that CDK inhibition may overcome PARP resistance.

The minimally invasive single port robot-assisted radical prostatectomy led to high postoperative continence rates and significant improvements in IPSS and PVR measures.
"The device is noninvasive and used in awake subjects. This case demonstrates that even chronic fragments can be successfully cleared, sometimes within hours of the procedure, even if they have not previously cleared for months,” said Mathew Sorensen, MD.

The researchers did identify several other factors that were independent predictors of cancer-specific mortality in this patient population.

The combination demonstrated encouraging clinical activity in patients with both clear cell and non–clear cell renal cell carcinoma.

The novel oncolytic immunotherapy CG0070 is also currently being explored as a in a phase 3 monotherapy trial.
Over half of patients had pathologic downstaging after receiving the neoadjuvant regimen.
“We found that the elderly, female, and patients with Medicaid insurance had the highest decreases in outpatient visits, and among the lowest magnitude recovery compared to pre-pandemic levels," the authors wrote.

Using stereotactic body radiotherapy to reduce the number of treatment sessions with fewer, more intense doses did not lead to an increase in gastrointestinal or genitourinary acute toxicity.

"This is the first time that I have seen such impressive responses with an immunotherapy product. The responses of my patients in the trial are far beyond my expectations," said study investigator Susan F. Slovin, MD, PhD.

The FDA approved the ready-to-use 6-month subcutaneous depot formulation of leuprolide mesylate earlier this year for the treatment of patients with advanced prostate cancer.

The FDA has converted the accelerated approval of frontline pembrolizumab in advanced bladder cancer to a full approval and revised the indication to cover the treatment of patients with locally advanced or metastatic urothelial carcinoma who are not eligible for any platinum-containing chemotherapy.
Nonsurgical primary chemoablation with the mitomycin-containing reverse thermal gel UGN-102 showed promise as an alternative to repetitive surgery for patients with low-grade non–muscle invasive bladder cancer.
The theranostic 64/67Cu SAR-bisPSMA combines the PSMA PET imaging product 64Cu-SAR-bisPSMA and the PSMA targeted therapy 67Cu-SAR-bisPSMA.

Ventana MMR RxDx Panel is a qualitative immunohistochemistry test that assesses a panel of MMR proteins to help guide clinicians in their treatment decisions.

Combining the Stockholm3 blood test with MRI-guided prostate biopsy reduced the number of MRIs performed and lowered overdetection, while not sacrificing the capacity to identify clinically significant tumors, according to findings from the STHLM3-MRI study.

The approval provides urologists with another tool for the treatment of patients with genitourinary cancers and underscores the rapidly growing significance of genetic testing in the field of urology.

Preclinical data have provided further evidence that adding the novel radiosensitizer idronoxil to 177Lu-PSMA-617 may improve survival in patients with metastatic castration-resistant prostate cancer.