Prostate Cancer
Latest News
Latest Videos

CME Content
More News
The addition of the AKT inhibitor ipatasertib to abiraterone acetate and prednisone improved radiographic progression-free survival, with overall survival data still eagerly anticipated.

A new drug application has been submitted to the FDA for TLX591-CDx, a radiopharmaceutical cold kit for the preparation of 68Ga-PSMA-11 injection for PET imaging in prostate cancer.

Treatment with AMG 160 showed a manageable safety profile with preliminary efficacy in patients with metastatic castration-resistant prostate cancer.

The PARP inhibitor reduced the risk for death by 31% in men with metastatic castration-resistant prostate cancer, compared with enzalutamide or abiraterone plus prednisone.

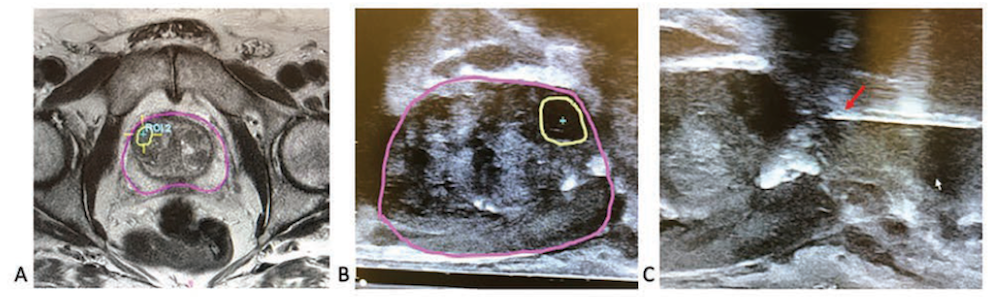
Based on the findings, investigators recommended that systematic biopsy be performed in patients with negative MRI and in addition to targeted biopsies in men with a positive MRI.

The FDA has granted fast track designation to EPI-7386, an oral, highly selective N-terminal domain inhibitor of the androgen receptor for treatment of mCRPC resistant to standard-of-care treatment.

Updated results were also reported on other key end points, including time to pain progression, first symptomatic skeletal event, and initiation of chemotherapy.
Combination approach could overcome mechanisms that previously led to poor outcomes with single-agent checkpoint inhibition.

Advances in receptor targeted PET imaging continue to refine the identification of metastatic disease.

A new analysis supports return to widespread screening.

The results suggest the minimally-invasive procedure is an acceptable alternative to immediate surgery or radiation.
Mark C. Markowski, MD, PhD, and Emmanuel S. Antonarakis, MD, provide potential explanations for the increased clinical activity of PARP inhibitors in BRCA2- versus BRCA1-mutated prostate cancer.

Personalized approach would reduce the number of biopsies some patients need to receive.

The overall risk of cardiac disorders was higher with abiraterone versus enzalutamide.

Treatment with the pan-BET bromodomain inhibitor ZEN-3694 plus enzalutamide may re-sensitize patients to androgen receptor–targeting agents.

Survival outcomes are not compromised in men undergoing active surveillance, a recent study finds.

The FoundationOne Liquid CDx pan-tumor liquid biopsy examines over 300 cancer-related genes in a patient’s blood sample to inspect for deleterious alterations.

In this episode, Samuel J. Peretsman, MD, discusses how to get started with HIFU for prostate cancer and performing robotic prostatectomy following HIFU.

Focal therapy using HIFU may be offered as an alternative to the existing modalities of treatment for select patients with all risk profiles of prostate cancer.

Starting radiotherapy up to 6 months following the start of androgen-deprivation therapy was not linked to poorer overall survival outcomes.

Next-generation sequencing identifies subtypes that may benefit from immune checkpoint inhibitors.

Findings from the phase 2 TRITON2 trial, which led to the FDA approval of the PARP inhibitor rucaparib in metastatic castration-resistant prostate cancer, have been published in the Journal of Clinical Oncology.

Against the backdrop of a new guideline for advanced prostate cancer, Robert A. Dowling, MD, describes how you might measure adherence to guidelines in your own practice.

Response and survival rates with the immunotherapy-based regimen support ongoing phase 3 research with pembrolizumab in prostate cancer.

Agent shows statistically significant superiority to conventional computed tomography.